Diffusion of gases
The applet below compares the diffusion of two gases at a chosen temperature.
- diffusion is the spreading out of one substance through another substance. The direction of diffusion is from a region of high concentration to a region of low concentration.
|
Thomas Graham (1805-1869), a scottish chemist and early physical chemist, studied the rate at which a gas diffused from a region of high concentration to a region of low concentration through small holes or pores. A law of effusion rate was developed from his studies.
- effusion is the escape of one substance through a small hole.
|
Graham determined that the rate of effusion of a particular gas at constant temperature and pressure was inversely proportional to the square root of its molar mass. The diffusion rate of a gas has also been shown to approximately follow this relationship.
- r, the rate of diffusion is approximately inversely proportioanl to the square root of the molar mass, M.
r  1 / sqrt(M) 1 / sqrt(M)
- it follows that for two gases with molar masses M1 and M2, the two respective rates of diffusion, r1 and r2, are related to the two molar masses by the following relationship:
r1 / r2 = sqrt (M2 / M1)
|
The applet below can be used to confirm the above relationship by calculating a diffusion rate from the the relative distance travelled to the first collision between two different gas particles and the time elapsed to this first collision:
diffusion rate (s-1) = relative distance travelled / time elapsed to first collision (s)
The applet also displays a mean speed for each gas. The applet can also be used to confirm the relationship between temperature and mean speed of the gas:
mean speed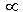 sqrt (T / M)
sqrt (T / M)
Where T is the absolute temperature.
It follows from the last expression that doubling the temperature of any gas, say from 400 K to 800 K, increases the mean speed of its particles by sqrt(2) = 1.4.
- The initial conditions for the model are for both gas A and gas B to be neon at a temperature of 273 K.
|
Notes on using the applet:
- the particles in the model have a range of velocities.
- no reactions are shown between colliding particles.
- the model only shows 50 particles of each gas. This very small number of molecules compared to near molar amounts in real experiments means that errors are larger in the model than in much empirical work. These errors may be reduced in the normal way by making replicate measurments from the model.
- the applet automatically stops running after 5 minutes.
|
 1 / sqrt(M)
1 / sqrt(M)