Chemistry
Ionic radii
Like the atomic radii, ionic radii are determined by measuring the distance between adjacent nuclei. However, for ions this gives the sum of the ionic radii for an anion/cation pair and so in practice the radius of the oxide anion, O2-, at 146 pm is used as a reference point from which to calculate other ionic radii.
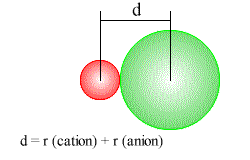
- The ionic radius of an element is its share of the distance between adjacent ions in an ionic solid.
|
For example, the distance between the magnesium cation and the oxide anion in magnesium oxide is 211 pm, the radius of the Mg2+ ion is calculated from 205pm - 146 pm = 65 pm.
The size of an ion also varies somewhat with its environment so average values for ionic radii are often quoted.
One way in which the ionic radii of the elements can be compared is to compare ions that are isoelectronic: that is, ions which have identical electron configurations. The table below compares the electron configurations of element atoms and their isoelectronic ions across period 3.
Cations
| Element |
Sodium |
Magnesium |
Aluminium |
| Electron configuration of atom |
[Ne] 3s1 |
[Ne] 3s2 |
[Ne] 3s2 3p1 |
| Ion |
Na+ |
Mg2+ |
Al3+ |
| Electron configuration of ion |
[Ne] |
[Ne] |
[Ne] |
Anions
| Element |
Silicon |
Phosphorus |
Sulphur |
Chlorine |
| Electron configuration of atom |
[Ne] 3s2 3p2 |
[Ne] 3s2 3p3 |
[Ne] 3s2 3p4 |
[Ne] 3s2 3p5 |
| Ion |
Si4- |
P3- |
S2- |
Cl- |
| Electron configuration of ion |
[Ar] |
[Ar] |
[Ar] |
[Ar] |
You can now use the Radii Explorer applet below to explore the difference in ionic and atomic radii across a period and down a group. Ionic radii are for ions isoelectronic with a noble gas as described above with, for example silicon as Si4- and hydrogen as H-.
You should use the applet to find out:
- the relative size of the radius of a cation compared to the parent element atom
- the relative size of the radius of a anion compared to the parent element atom
- the trend in ionic radii across period 3
- the trend in ionic radii down either the alkali metal or the halogen group
You should have discovered that:
- cations are always smaller than their parent element atoms, because the atom loses one or more electrons to form the cation and exposes its core which is generally much smaller than than the parent atom.
- anions are always larger than their parent element atoms, because the atom gains one or more electrons to form the anion. The increased number of electrons in the valence shell increases the repulsive effects that the electrons exert on one another, thus increasing the radius.
- the trend in ionic radii across period 3 shows a decrese for the cations of Na, Mg, and Al as not only do the atomic radii of the parent element atoms decrease across the period, but the [Ne] core that is exposed when the ions form is attracted progessively more strongly by the increasing nuclear charge.
- the trend in ionic radii across period 3 shows a decrese for the anions of Si, P, S, and Cl as each ion has an electron configuration isoelectronic with argon but a progessively larger nuclear charge exerting a greater electrostatic force of attraction on the valence shell electrons.
- the trend in ionic radii down a group shows an increase for the same reasons as atomic radii increase down a group.
|

